, Podcast, Selling Hay
Seeding Success: a discussion with Grower Relation | RSS.comPodcast Highlights This week, Jon Paul Driver chats to Grower Relations Manager Stephen Page about the recent rains, the upcoming hay season, shifts we’re seeing in bale contracting, and opportunities for spreading risk by working to the conditions. Episode Highlights: A variety…
Read More
Tips For A Profitable Hay Season 2023/24
, Selling Hay
Ready for new Hay Season? After some unpredictable weather conditions over the last few years, we’re all excited for a return to good hay-growing conditions. That’s why we’ve put together a practical guide to help you navigate the road to success in the upcoming hay season. There are many steps…
Read MoreFeed Central Celebrates 21 Years!
0
Dear Feed Central Community, Feed Central celebrated its 21st birthday this week! It’s hard to believe that it’s been 21…
Everything You Need To Know About Potassium and Horses
Potassium is a mineral that is found in many foods, and it is also necessary for horses. It plays a…
How Potassium Affects Dairy Cattle
For lactating dairy cattle, potassium is an imperative nutrient that optimizes milk production. The Nutrient Requirements for Dairy Cattle from…
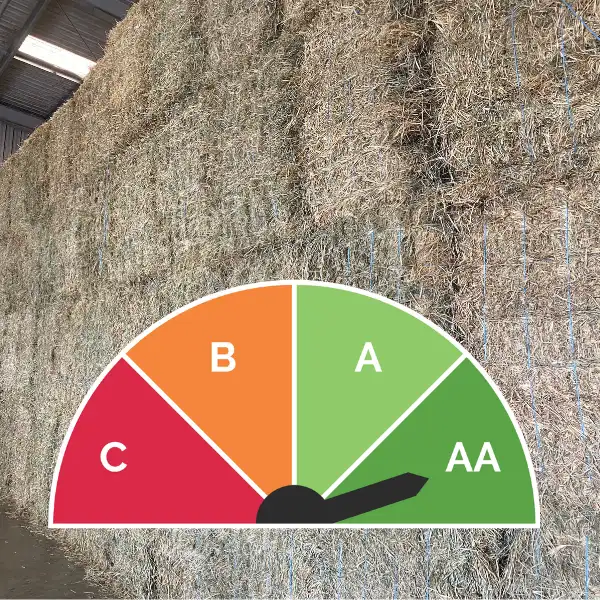
The Visual Gauge And How It Works
Why Is Visual Grade Important?Visual Grade has a very strong correlation to the attractiveness of the hay (for the livestock),…
We are proudly Australia’s largest buying and selling platform for hay. We’ve invested years developing our Quality Assurance and Inspection system that is now recognised Australia-wide. With over 20 years of working within the agricultural industry, Feed Central offers an online system backed by quality assurance and real people, whether you’re buying, selling or testing hay – Feed Central is here to help.